Supreme Court verdict announced on Thursday against A challenge to the Food and Drug Administration’s (FDA) regulatory approval process for the abortion drug mifepristone, the latest abortion case since the landmark decision overturning Roe v. Wade in 2022.
For a victory Biden administration And in the case of abortion rights supporters, the high court ruled unanimously that challengers to the FDA did not have the standing to sue the government.
Justice Brett Kavanaugh, who authored the unanimous opinion, wrote, “Under Article III of the Constitution, plaintiffs’ desire to make a drug less available to others does not establish a basis to sue. Nor are plaintiffs’ other grounds theories sufficient.”
“Plaintiffs have serious legal, moral, ideological, and policy objections to the FDA’s lax regulation of elective abortion and mifepristone,” he said. “But under Article III of the Constitution, such objections alone do not establish a justiciable case or controversy in federal court.” The case was remanded back to the Fifth Circuit, consistent with the Court’s opinion.
Key study in FDA abortion pill case before Supreme Court was withdrawn in ‘partisan attack’: author
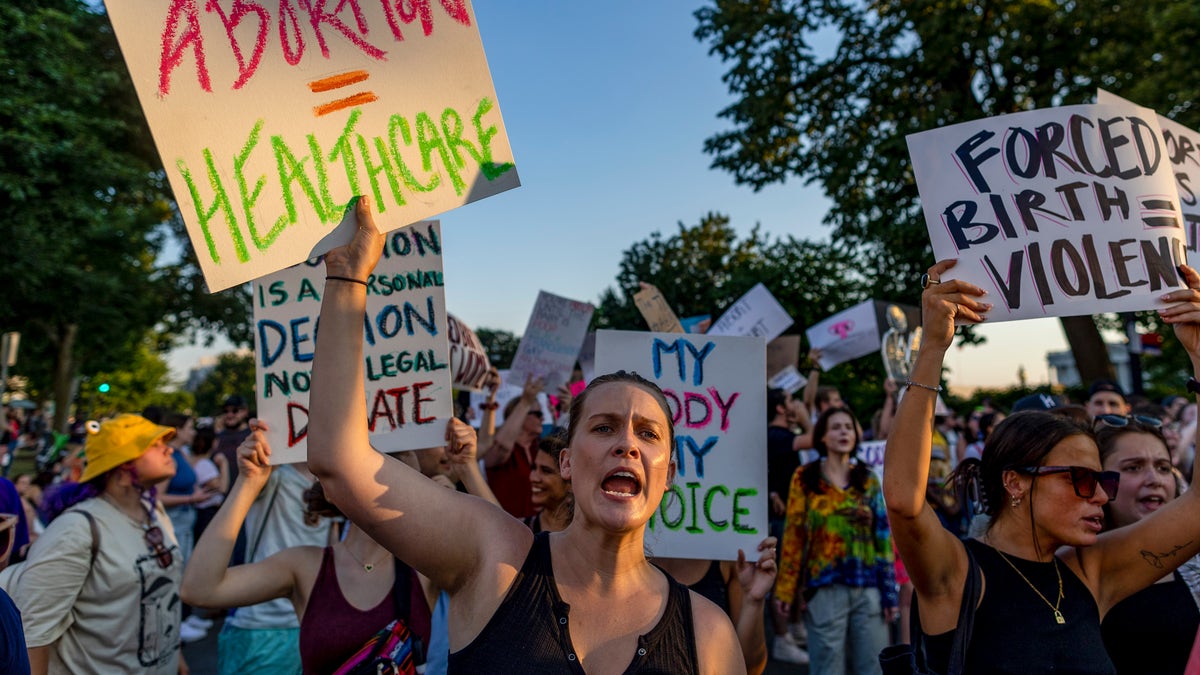
Protesters gather outside the US Supreme Court on June 25, 2022 in Washington, DC, in the wake of the decision to overturn the Roe v. Wade case. (Photo: Tassos Katopodis/Getty Images)
The case arose from lawsuits filed by the Alliance for Hippocratic Medicine, a group of health care associations, which claimed the drug caused an excessively high rate of complications.
“We are disappointed that the Supreme Court could not decide the FDA’s illegal removal of normal safety standards for abortion drugs,” said Erin Hawley, an attorney with the civil rights firm Alliance Defending Freedom, which fought the case against the FDA.
He said, “Today’s decision does not change the fact that the FDA’s own label states that approximately one in 25 women who take chemical abortion drugs will end up in the emergency room — an alarming reality that the doctors and medical associations we represent in this case know all too well. The FDA leaves women and girls to take these high-risk drugs alone in their homes or dormitories without the constant, in-person care they need from a doctor.”
They added, “While we are disappointed by the court’s decision, we will continue to advocate for women and work to restore common safeguards for abortion drugs — like early office visits to check for ectopic pregnancies. And we are grateful that three states are willing to hold the FDA accountable for endangering the health and safety of women and girls across this country.”
The Supreme Court said the group could not prove that loosening the FDA’s rules would have caused them any harm, giving them sufficient grounds to sue.
He added, “Here, plaintiffs have failed to demonstrate that they would actually suffer harm as a result of the FDA’s lax regulatory requirements. For this reason, federal court is the wrong forum to address plaintiffs’ concerns about the FDA’s actions.”
“Plaintiffs can present their concerns and objections to the President and the FDA in the regulatory process, or to Congress and the President in the legislative process. And they can also express their views about abortion and mifepristone to fellow citizens, including in the political and electoral processes,” they wrote.
In March, the justices heard arguments for about 90 minutes about federal government rules from 2016 that made it easier to access mifepristone, including access by mail.

Boxes of the drug mifepristone sit on a shelf at the West Alabama Women’s Center on March 16, 2022 in Tuscaloosa, Alabama. (Getty Images)
In Overturning Roe v. Wade In June 2022, the Supreme Court ruled in Dobbs v. Jackson Women’s Health Organization that the US Constitution does not guarantee a right to abortion and that the matter could be decided by the states.
Subsequently, 14 states ban abortion at all stages of pregnancy with some exceptions, and two others ban abortion once a fetal heartbeat is detected, which occurs after about six weeks of pregnancy.
Mifepristone is taken with misoprostol, and the combination of the two drugs is known as medication abortion or “mifepristone.”Abortion Pill,
Lower courts concluded that the federal agency did not fully consider the potential health risks to women when it revised regulations for mifepristone in 2016. Those revisions — last updated in 2023 — included lowering the recommended dosage, allowing the drug’s use up to 10 weeks of pregnancy (from seven weeks), approving a generic version and allowing it to be mailed (eliminating the need to visit a doctor in person), among other measures.
The Biden administration and the manufacturer of mifepristone urged the court to overturn the appellate decision, which would end access to the drug by mail and impose other restrictions, even in states where abortion is legal.

A crowd of supporters gathers outside the Supreme Court in Washington on Friday, June 24, 2022. (AP Photo/Jose Luis Magana)
The restrictions include reducing the current period of use of mifepristone in pregnancy from 10 weeks to seven weeks.
According to data from the Guttmacher Institute research group, about two-thirds of all abortions in the US in 2023 relied on mifepristone. Nearly six million women have used the drug since its approval 24 years ago.
A spokesman for Danco, the drug’s manufacturer, said: “We are pleased with the Supreme Court’s decision in this extremely important case.”
“By rejecting the Fifth Circuit’s radical, unprecedented and unsupportable interpretation of the right to sue, the justices have reaffirmed longstanding fundamental principles of administrative law,” Long said.
“In doing so, they maintained the stability of the FDA drug approval process, which is based on the agency’s expertise and is trusted by patients, health care providers, and the U.S. pharmaceutical industry,” he added.
Click here to get the Fox News app
“We thank the Court for its careful analysis and remain committed to developing and bringing to market safe and effective products in this important area of public health.”
Justice Clarence Thomas, in his concurring opinion, highlighted his concerns regarding the “ally position” raised in the case.
He said, “The Alliance’s attempt to use our association-standing doctrine demonstrates how far we have departed from the traditional rule that plaintiffs should assert only their own injuries. The Alliance is an association whose members are other associations. None of its members are doctors. Instead, the Alliance seeks to maintain the association’s standing based on injuries to doctors of its member association members. Thus, the allegedly injured parties – the doctors – are two degrees of separation from the parties before us pursuing those injuries.”

















